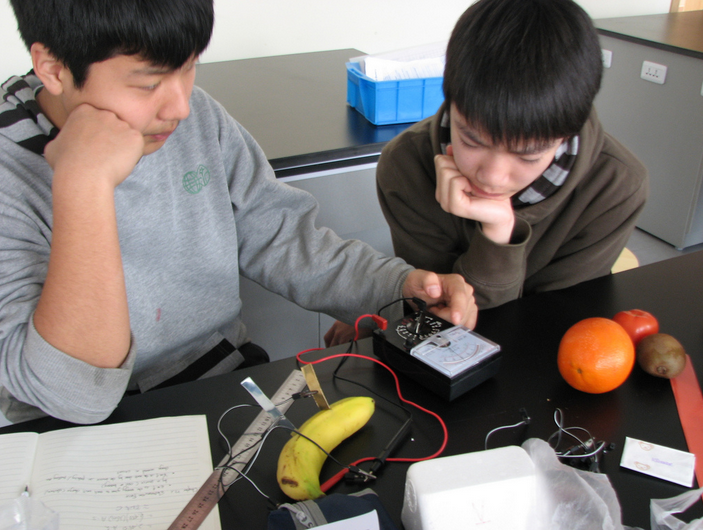
A Quick and Easy Science Fair Project Idea – Fruity Battery
Most of our favorite things like toys, cars, phones, watches and speakers need batteries to survive! The devices stop working and are termed dead when their batteries exhaust. So, why don’t you learn to make a battery at home with a few simple supplies and understand its functionality with this cool science fair project idea?
Photo Courtesy: Richard Heaven
Question/Problem
Functionality of a battery
Supplies
An orange
18 gauge copper wire
Clipper
Steel paper clip
A piece of zinc
Sandpaper
Adult’s assistance
Directions
Use a clipper to take off about 2 1/2 inches of plastic insulation that’s found on the copper wire. Now, clip the loose piece off the main roll.
Use all your strength to straighten the steel paper clip. Ask an adult for assistance to help you straighten it. Measure it at 2 1/2 inches and cut off the remaining.
Rub off any rough spots in the wire or the paperclip with sandpaper. Make sure it’s smoothened perfectly.
Now, roll the orange in your hand to loosen up the juice inside. You can also roll it on a flat surface to break the cell walls as you will need the juice to form the foundation of this science fair project.
Stick the copper wire about 1 inch into the orange. Moisten your tongue with saliva and lightly touch the wire. Do you notice any distinct taste?
Now, carefully stick the paper clip and zinc strip into the orange about 1/4 inch next to the wire making sure no metal touches another. Again, touch the wire ends with your tongue. What do you notice this time?
Observations
There must have been a distinct difference in taste when you touched your tongue to the copper wire and then all the metals and alloys. While just the copper wire was plain, the metals and alloy were metallic to taste.
Explanation
Why did you feel the metallic taste?
The orange battery was generating electricity which means the electrons from the wire were travelling to your tongue. The electrons moved across the surface of the tongue and hence you got the metallic taste. Electrons are smaller breakdowns of an atom and are electrically charged.
The kind of battery that you made is called a voltaic battery. It is made of two different metals which act as electrodes, passages from where any current is transferred. Since electrolytes(sour acid from the orange and the saliva) transfer electricity when dissolved in water, you felt the tingling taste because your mouth was moist, as was the orange. Batteries stop working when there aren’t enough electrolytes to react with metals or not enough metals to react with the electrolytes.
Experimenting with the science fair project idea
· Connect multiple orange batteries to generate more electrical current. Use a copper wire to connect the two as a bridge.
Photo Courtesy: Micah Sittig
· Use variables and repeat the science fair project idea with a variety of acidic fruits and vegetables and measure which one generates most current.
Author Bio:
Catherine Ross is a full-time stay-at-home-mum who believes learning should be enjoyable for young minds. An erstwhile elementary school teacher, Catherine loves coming up with creative ways through which kids can grasp the seemingly difficult concepts of learning easily. She believes that a ‘fun factor’ can go a long way in enhancing kids’ understanding and blogs at http://kidslearninggames.weebly.com/


Hello, i think that i saw you visited my website thus i came to “return the favor”.I’m trying to
find things to enhance my website!I suppose its ok to use a few of your ideas!!